

根據消息,歐盟負責食品藥品安全機構歐洲安全聯盟ESF在近期發布了官方聲明與警告!
歐洲安全聯盟ESF從不同的來源獲悉:
有關用作PPE CE標記基礎的“證書”(包括FFP2 / FFP3口罩和護目鏡),這些”認證“系偽造或者非授權所發,不能用作合格證明,不具備法律價值,并指出,任何進口的產品必須要提供Doc(即檢查合格證明)。
ESF警告內容:
Unfortunately, we are informed from different sources about ‘certificates’ used as basis for CE marking of PPE (including FFP2/FFP3 masks and eye protection), while these ‘certificates’ have no legal value and can not be used as conclusion of conformity assessment. It is not clear if these documents have actually been issued by the organisations mentioned themselves or if they are fake.....
根據(EU)2016/425法規,防護口罩(如FFP2 / FFP3)屬于III類PPE。
這意味著合格評定包括:
1. 由公告機構(Notified Body)進行的型式檢驗,合格后獲發“EU型式檢驗證書”,簡稱Module B證書。
2. 由公告機構進行的生產跟蹤或隨機抽查或體系審核,簡稱Module D證書或Module C2證書。
只有獲得Module B+ Module D或Module B+ Module C2證書后,才可使用CE標識,標識旁顯示公告機構的編號。該聲明必須與產品使用說明隨附(或說明可查詢網址)。
同時在公告中,歐洲安全聯盟ESF還列舉了21家被判不合規的認證組織和機構,名單中也包括了我們國內的機構
其中被點名的國內認證機構如下
●CIC (Shenzhen CIC Testing Technology)
●Huaxun (Shenzhen HX ●●Detect Certification)
●ENC (East Notice Certification Service)
●HTT (Shenzhen HTT Technology)
●ITC (Shenzhen ITC Product Testing)
●BTK (Guangzhou Bestek Testing Services)
●Micez (Shanghai MICEZ Testing & Technical)
●Huawin (Shenzhen Huawin Testing Certification)
●LTT (Shengzhen LTT Testing Technology)
●AST.LAB (Aerospace Testing Technology (Shenzhen)
被點名的歐洲認證機構名單如下
●ICR Polska - see update 31/03/2020 and 06/04/2020 below
●CELAB - see statement on their webpage https://celab.com/en/coronavirus/
●ISET (Instituto Servizi Europei Technologici) - on their website they have a page with false certificates - see http://www.iset-italia.eu/index/service/fal.html
●ECM (Ente Certificazione Macchine) - also a picture of a mask with identification number of the notified body ECM 1282 next to CE - ECM is not a notified body for PPE, so this marking is certainly not valid) - see update 03/04/2020 below
●NPS
●Amtre Veritas
●STS Inspection and Certification
●VIC Testing and Certification
●BSI : we have also added an example of a 'certificate of compliance' issued by 'BSI, London' which is clearly not issued by the Notified Body for PPE BSI and this is confirmed by the Notified Body BSI - so this one is not a valid EU Type Examination certificate.
我們(ESF)還添加了一個由“倫敦BSI”頒發的“合規證明”的示例,該例子顯然不是由PPE BSI的公告機構簽發的,并且得到了公告機構BSI的確認-因此,這是一個無效的歐盟類型檢驗證書。
●BSI : we have an example of a BSI EU Type Examination Certificate that has clearly been changed and is thus a fake document - this is confirmed by the Notified Body for PPE BSI.
我們有一個BSI歐盟類型檢驗證書的示例,該證書已明顯更改,因此是偽造文件-由PPE BSI公告機構確認。
被點名的2家不合規機構
●Nova Certification (based in Greece) - the Notified Body Nova (not notified for PPE assessment but for other types of products) confirmed that the example of the 'declaration of conformity' is a fake document
-新產品公告機構(未進行PPE評估,但針對其他類型的產品進行通知)確認“符合性聲明”示例為偽造文件
●GTS (Global Testing Services, based in China)
歐洲安全聯盟ESF(European Safety Federation)官方提供被點名企業或機構的不合規樣板











(圖片來自搜航網整理)
既然歐盟CE認證的假證如此泛濫,那要如何判斷真假呢?
小編為大家整理一些資料,供大家參考
口罩制造和出口企業如果要申請認證,首先要問發證機構兩個問題:
1、貴司是否為NB機構? 機構號是否可以查詢?
2、出具的CE證書在官網可查嗎?

(常見的CE認證偽證書,圖片海關發布)
歐盟公布了一系列由歐盟統一監管和認證資質授權的機構,也就是我們說的NB機構,并授予每家機構一個唯一的四位數編碼即公告號,CE證書的申請和頒發就由對應法規和指令授權的公告號機構頒發。
鏈接如下
https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=notifiedbody.main
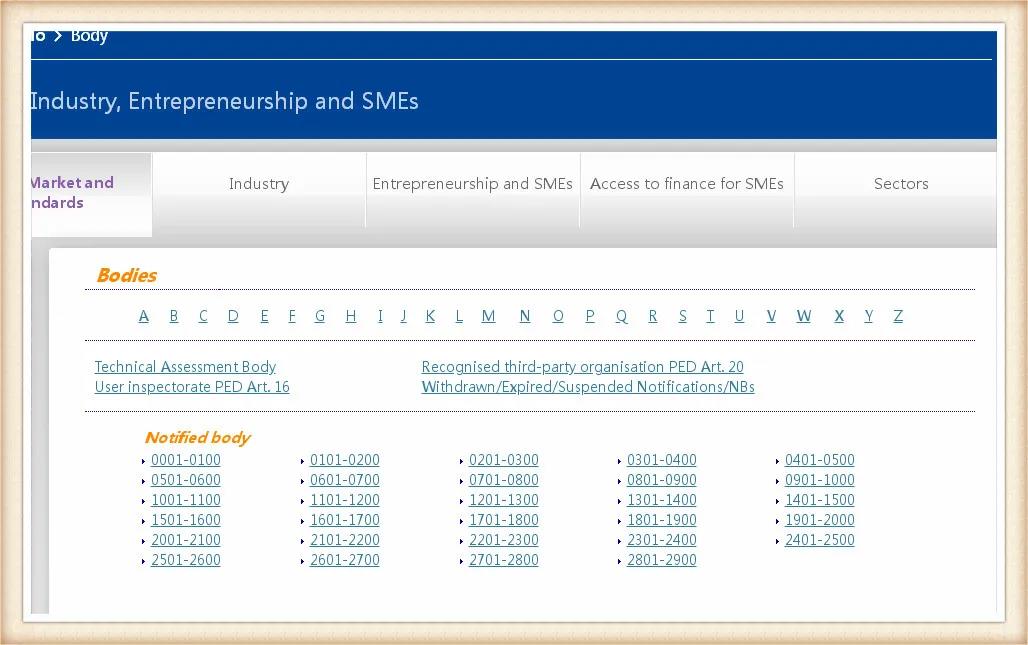
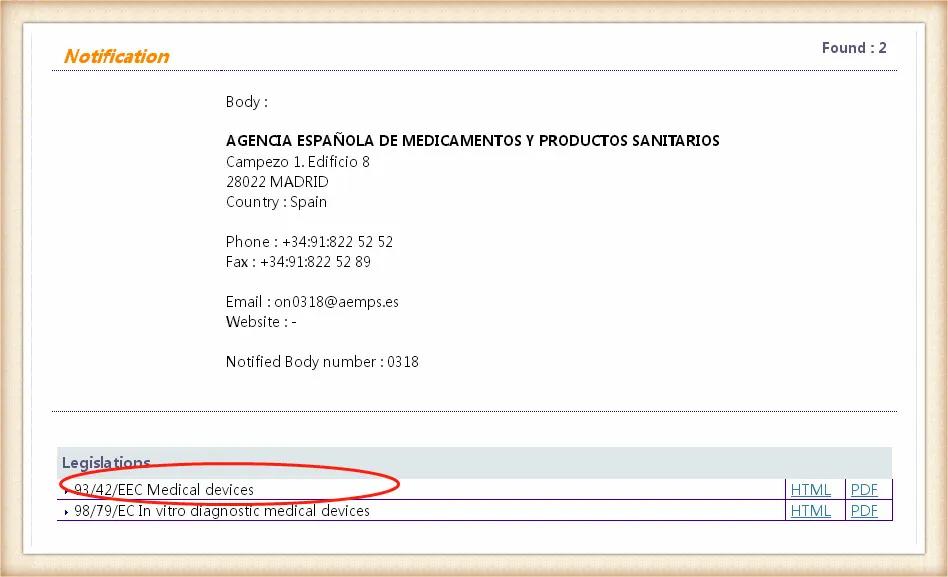
對應所獲取的NB授權號,點擊相應的編碼所在位置,進入以后可以查詢該機構得到授權的指令。出具的在授權范圍內的指令的認證證書才是有效的。
目前歐盟和口罩相關的指令有:醫療器械指令93/42/EEC(MMD)、醫療器械的新法規(EU) 2017/745(MDR)、個人防護裝備(PPE)法規(EU)2016/425。
(來源:小馬看跨境電商)
以上內容屬作者個人觀點 ,不代表雨果網立場!如有侵權,請聯系我們請聯系我們 。